
Tuesday, 20 March 2018  |
 |
|
Experts explain their approach to treating patients who are living longer with cancer that has spread to the spine, as the options for metastatic spine tumours increase. Every kind of cancer can spread to the spine, yet two physician-scientists who treat these patients describe a paucity of guidance for effectively providing care and minimising pain.
|
|
 |
|
Scientists at Cincinnati Children’s Hospital Medical Centre report an experimental molecular therapy that restores insulation around peripheral nerves in mice, improves limb function, and results in less observable discomfort. These results were initially described in Nature Medicine in February 2018.
|
|
 |
|
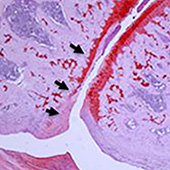
A team lead by Denis Evseenko (Department of Orthopaedic Surgery, University of Southern California, Los Angeles, USA) hopes to delay or reduce the need for joint replacement surgery in osteoarthritis patients with an injection. When osteoarthritis worsens, joint replacement surgery is often the only option for patients.
|
|
|
 |
 |
|
Camber Spine, a leading innovator in spinal and medical technologies, has announced the first surgeries using the company’s proprietary SPIRA-C Open Matrix Cervical Interbody device, a unique, interbody fusion implant consisting of spiral support arches and Surface by Design technology. The first case using the Spira-C took place on 8th January 2018.
|
|
 |
|
Spinal muscular atrophy (SMA) patients treated with SPINRAZA (nusinersen) have experienced stabilised or improved motor function, contrary to the natural course of the degenerative disease, Biogen and Ionic Pharmaceuticals have announced. This is the end of study result from CHERISH, the phase 3 study comparing the use of nusinersen with a sham control for SMA treatment, recently published in The New England Journal of Medicine.
|
|
 |
|
Spineology has announced that enrolment is now complete in the company’s Spineology Clinical Outcomes Trial (SCOUT) clinical trial. The SCOUT IDE, conducted under an FDA-approved protocol, is a prospective, multicentre non-randomised performance goal investigation, designed to evaluate safety and effectiveness outcomes in instrumented lumbar interbody fusion procedures for the treatment of degenerative disc disease.
|
|
|
NSpine 2019 preparations are underway
NSpine preparations are underway for summer 2019, which will again see an extensive programme covering all aspects of spinal healthcare. The Institution of Engineering and Technology at Savoy Place, London, UK has been chosen as the venue, providing the ideal backdrop for the additional free papers on surgical simulation, active robotics and artificial intelligence in spinal surgery.
|
|
|
|
|
You are receiving this email because you subscribed/registered on www.spinalnewsinternational.com
Our mailing address is:
BIBA Medical Ltd, 526 Fulham Road, Fulham, London, SW6 5NR
TEL: +44 (0)20 7736 8788
FAX: +44 (0)20 7736 8283
EMAIL: info@bibamedical.com
© BIBA Medical Ltd is a company registered in England and Wales with company number 2944429. VAT registration number 730 6811 50
|
|