
Friday, 25 May 2018  |
 |
 |
|
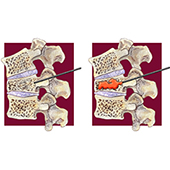
Percutaneous vertebroplasty does not result in statistically significant greater pain relief than a sham procedure, reports a study detailing the results of the VERTOS IV trial, published in the British Medical Journal (BMJ). This study followed patients with acute osteoporotic vertebral compression fractures for 12 months following either a vertebroplasty or a sham intervention.
|
|
 |
|
Auto-registration is better than point-to-point registration with respect to clinical accuracy when using the same active infrared navigation system during spinal surgery. This is the conclusion of the first study to compare the difference between the two methods with respect to clinical accuracy, originally published in Spine.
|
|
 |
|
Michael Jackson’s gravity-defying Smooth Criminal dance move, which wowed live audiences and inspired new forms of dancing, was down to core strength and an illusion, neurosurgeons write in The Journal of Neurosurgery: Spine. Dubbed the anti-gravity tilt, the seemingly impossible dance move was first showcased in the 1987 music video for Smooth Criminal.
|
|
|
 |
|
|
|
Bruce Dall is a spine surgeon and an advocate for those with sacroiliac joint pain, publishing multiple papers on the joint. Here, he argues that the global spinal scene has been neglecting the sacroiliac joint for too long, and that patients are suffering as a result.
|
|
|
 |
|
Spinal Elements, a spine technology company, has announced the release of its Clutch interspinous process device. This new product further enhances the breadth of Spinal Elements’ thoracolumbar portfolio and offers surgeons more options for treatment of various posterior thoracolumbar pathologies, a press release states.
|
|
 |
|
DiscGenics, a clinical stage regenerative medicine company, has announced the first patient has been treated in its phase I/II US clinical trial of IDCT for mild-to-moderate degenerative disc disease. The treatment took place at Carolina Neurosurgery and Spine Associates in Charlotte, USA, led by the study’s principal investigator, Domagoj Coric (Charlotte, USA).
|
|
 |
|
Infuse Bone Graft (Medtronic) has gained US Food and Drug Administration approval in new spine surgery indications. InfuseBone Graft is now approved for use with additional spinal implants made of polyetheretherketone in oblique lateral interbody fusion and anterior lumbar interbody fusion procedures at a single level. This is the second expanded indication in just over two years.
|
|
|
|

