
Friday, 13 January 2016  |
 |
|
Live-imaging and transcriptome analysis of medaka fish transgenic lines has revealed that exposure to microgravity can lead to the immediate alteration of cells responsible for bone structure formation.
|
|
 |
|
Fewer veterans received prescriptions for risky dosages of opioid painkillers after a USA-wide initiative took aim at reducing high doses and potentially dangerous drug combinations, a new study has found.
|
|
|
|
|
|
The US Food and Drug Administration (FDA) has formally reclassified pedicle screws from class III to class II devices. The reclassification, supported by NASS, SRS, came into force on 20 December 2016.
|
|
|
|
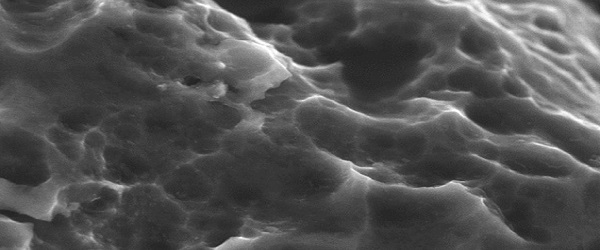
Surface technology for interbody fusion implants is an area of massive growth in the spinal market, with a large number of device companies and academic institutions hunting for the best ways to optimise bone/device integration. Spinal News International speaks to physicians Richard Guyer, Paul Slosar, Barbara Boyan and Sam Fang, about the latest developments in the field.
|
 |
|
Philips has announced the development of an augmented-reality surgical navigation technology, designed to help surgeons perform image-guided open and minimally-invasive spine surgery.
|
|
 |
|
DePuy Synthes Products has announced an asset purchase and development agreement with Interventional Spine. Financial terms of the transaction have not been disclosed.
|
|
|
|
|
|
The global market for spinal fusion—which includes spinal plating systems, interbody devices, vertebral body replacement devices, and pedicle screw systems (excluding minimally invasive spine devices)—is set to rise from approximately US$7.1 billion in 2016 to just under US$9 billion by 2023.
|
|
|
|



