
Friday, 24 February 2017  |
 |
|
Classification systems for conditions such as adult spinal deformity and spondylolisthesis increasingly use pelvic parameters as part of their calculations. Place and colleagues sought to investigate the reliability of accepting pelvic incidence as a “fixed” value by assessing its variability among healthy volunteers with no history of spinal abnormality.
|
|
 |
|
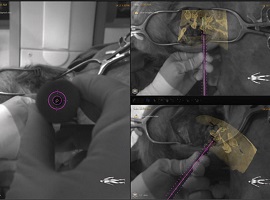
Philips’ new augmented reality system has been used in clinical practice for the first time at the Karolinska University Hospital, Solna, Sweden. A pre-clinical study published in Spine has demonstrated superior accuracy for thoracic pedicle screws placement using an augmented-reality navigation system (Philips) in comparison to those placed freehand.
|
|
|
|
|
|
In a value-based healthcare landscape, even when providing greater levels of evidence, surgeons increasingly face coverage denials from insurance companies for proven spinal procedures. Spinal News International explores the biggest issues facing spinal coverage in the coming months.
|
|
|
|
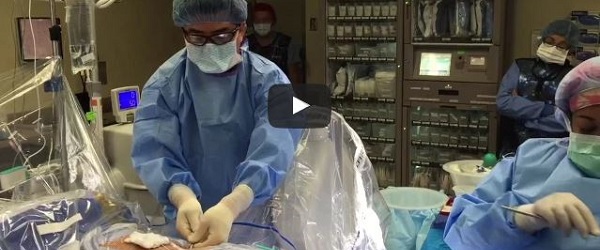
In this video shared by the Minimally Invasive Spine Centers of Excellence (MIS-COE), Choll Kim (San Diego, USA) takes the viewer through a live laser endoscopic spine surgery.
|
 |
|
The Balance ACS platform is designed to provide support to the full continuum of spinal care, from preauthorisation tools and preoperative planning to 3D anatomical modelling and postoperative reporting.
|
|
 |
|
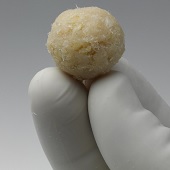
TranS1 has announced the release of the Capital bone graft harvester, a new device designed to allow for fast, reliable, and reproducible harvesting of autograft material from the iliac crest.
|
|
 |
|
DePuy Synthes, in collaboration with LifeNet Health, has launched ViviGen Formable cellular bone matrix, a second-generation cellular allograft.
|
|
|
|
|
|
|
You are receiving this email because you subscribed/registered on www.spinalnewsinternational.com
Our mailing address is:
BIBA Medical Ltd, 526 Fulham Road, Fulham, London, SW6 5NR
TEL: +44 (0)20 7736 8788
FAX: +44 (0)20 7736 8283
EMAIL: info@bibamedical.com
© BIBA Medical Ltd is a company registered in England and Wales with company number 2944429. VAT registration number 730 6811 50
unsubscribe
|
|