
Thursday, 9 March 2017  |
|
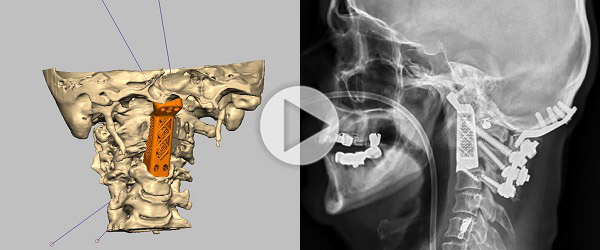
Ralph Mobbs from the Prince of Wales Hospital, Sydney, Australia, has replaced two cancerous vertebrae with 3D-printed implants, according to a report from the Australian Broadcasting Company (ABC). The world-first surgery took approximately 15 hours, and involved the separation and re-attachment of the patient’s skull from his spinal tissue.
|
 |
|
Patients diagnosed with lumbar degenerative spine disease are more likely to receive the right care when a team of experts representing multiple medical specialties collaborate in reviewing the patient’s needs and determining the best treatment option, research has shown.
|
|
 |
|
Published in the European Journal of Pain, a study of 4,390 Danish twins aged over 70 years old has found that those suffering from back pain had a 13% increased risk of all-cause mortality. The study investigated whether spinal pain increased the rate of all-cause and disease-specific cardiovascular mortality.
|
|
|
 |
 |
|
In response to new data shouwing that the use of computed tomography (CT) imaging following spinal surgery has increased significantly, Simplify Medical has suggested that its cervical disc could offer a solution.
|
|
 |
|
The French patent office has granted Implanet a patent protecting the Jazz Lock implant in the country. The device—a major component of the company's band platform—is an implant designed to treat degenerative spine disorders.
|
|
 |
|
Stryker has received US Food and Drug Administration (FDA) 510(k) clearance for its AVAflex balloon system. The device is available with the company's bone cements and implants and the AutoPlex mixing and delivery system.
|
|
|
|
|
|
|
15-17 March
BASS
Manchester, UK
12-14 April
ISASS17
Boca Raton, USA
|
|
|
|
|
|
|
You are receiving this email because you subscribed/registered on www.spinalnewsinternational.com
Our mailing address is:
BIBA Medical Ltd, 526 Fulham Road, Fulham, London, SW6 5NR
TEL: +44 (0)20 7736 8788
FAX: +44 (0)20 7736 8283
EMAIL: info@bibamedical.com
© BIBA Medical Ltd is a company registered in England and Wales with company number 2944429. VAT registration number 730 6811 50
unsubscribe
|
|